Novel, Smaller and More Powerful CTLA4 Inhibitors For The Treatment of Solid Tumors
At the American Chemical Society Spring 2021 Virtual Meeting & Expo, several Atomwise members and partners were selected to present their research and work. Learn what our Atoms have been working on below and visit Atomwise at ACS Spring 2021 Virtual Meeting & Expo for other presentation sessions.
 Navid Sobhani, PhD
Navid Sobhani, PhD
Baylor College of Medicine
Atomwise Collaborator: Aram Davtyan, PhD
Other Collaborator: Yong Li (Baylor College of Medicine)
Title: Novel, Smaller and More Powerful CTLA4 Inhibitors For The Treatment of Solid Tumors
Division: BIOL
Abstract
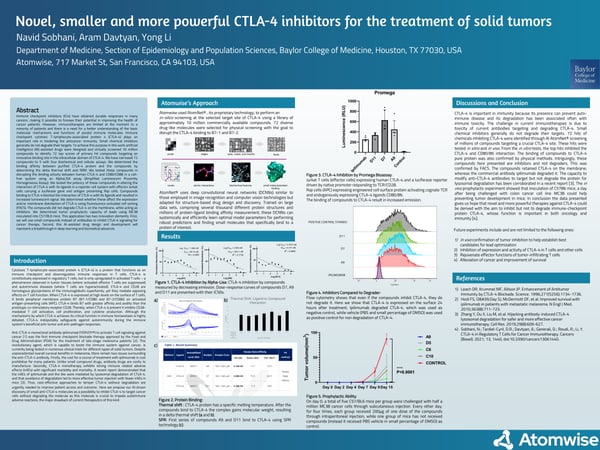
Immune checkpoint inhibitors (ICIs) have obtained durable responses in many cancers, making it possible to foresee their potential in improving the health of cancer patients. However, immunotherapies are limited at the moment to a minority of patients and there is a need for a better understanding of the basic molecular mechanisms and functions of pivotal immune molecules. Immune checkpoint cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) plays an important role in hindering the anticancer immunity. Small chemical inhibitors generally do not degrade their targets. To achieve this purpose in this work artificial intelligence (AI)-assisted drugs were designed and virtually screened 2.7 million compounds to identify 72 top scores of primary hit compounds targeting an innovative binding site in the intracellular domain of CTLA-4. We have narrowed 72 compounds to 5 with four biochemical and cellular assays. We determined the binding affinity between purified CTLA-4 protein and the compounds by determining the delta thermal shift and SRM. We tested these compounds in disrupting the binding activity between human CTLA-4 and CD80/CD86 in a cell-free system using an AlphaLISA assay (Amplified Luminescent Proximity Homogeneous Assay). We tested the potency of these compounds in altering the interaction of CTLA-4 with its ligands in a reporter cell system with effector Jurkat cells carrying a luciferase gene and antigen presenting Raji cells. Compounds binding to CTLA-4 blocked the interaction of CTLA-4 with its ligands and resulted in increased luminescent signal. We determined these compounds in affecting the expression and/or membrane distribution of CTLA-4 using fluorescence-activated cell sorting (FACS). The compounds did not degrade CTLA-4 on the membrane while acting as inhibitors. We determined tumor prophylactic capacity of leads using MC38 inoculated into C57/BL6 mice. This application has two innovation elements. First, we will use small compounds instead of antibodies to inhibit CTLA-4 signaling for cancer therapy. Second, this AI-assisted drug design and development will represent a breakthrough in deep learning and biomedical advances.
Poster Presentation
About AIMS
The AIMS Award program started in 2017, is designed to support promising researchers with resources that will help advance their work. AIMS Awards target research focused on finding solutions for complex human health conditions. To date, Atomwise has funded 7 rounds of AIMS Awards, completing over 100 collaborative projects and accepting over 775 projects into the program.
Related Posts
Subscribe
Stay up to date on new blog posts.
Atomwise needs the contact information you provide to send you updates. You may unsubscribe from these communications at any time. For information please review our Privacy Policy.